Regenerative potential of the intestine: even fully differentiated cells can become stem cells again
The intestinal epithelium forms the barrier between the organism and the environment and is thus exposed to a multitude of damaging factors. Severe injury, e.g. through a bacterial infection or toxic substances can compromise epithelial function. While most patients recover quickly, the disease can become chronic or even fatal for some. It is therefore important to understand the mechanism normally responsible for repairing the damaged epithelium. A team of scientists from the Charité University Medicine and the Max Planck Institute for Infection Biology in Berlin now showed that the colon epithelium can recover even if all stem cells and dividing cells die as a result of colitis - by converting differentiated cells into stem cells.
The mucosa of the intestine renews itself every seven days. This rapid cell turnover is driven by stem cells located in the base of the numerous intestinal crypts that continually generate new cells, which then undergo further cell divisions on their way to the surface, where they finally arrive as terminally differentiated cells. Once they reach the surface, they die within a few hours, only to be replaced by new cells from below. The team in Berlin now discovered in a mouse model that during an attack of colitis, stem cells in the crypts are selectively lost. They are however rapidly replaced by still dividing cells that have recently left the stem cell compartment, so that the epithelium again shows a normal structure shortly afterwards. During severe cases of colitis, however, these still dividing cells also died – leaving only the fully differentiated cells at the surface, while the rest of the crypts collapsed.
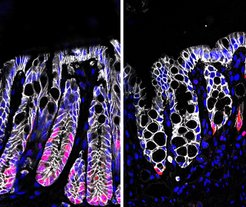
The base of deep crypts in the healthy colon (left) is occupied by dividing cells (pink). Crypts damaged during colitis (right) are less deep, the base with dividing cells is almost completely lost.
The team now showed that in this critical scenario the differentiated cells, which normally have a lifetime of only a few hours left, are reprogrammed to stem cells in order to regenerate a normal colonic epithelium. This reprogramming was driven by the signaling molecule R-spondin, which they recently showed to be produced by muscle cells beneath the epithelium, and which induces the expression of stem cell genes. “We could show that even differentiated cells still possess receptors that enable them to respond to R-spondin” confirms Michael Sigal, Gastroenterologist at the Charitè, who led the study in collaboration with the Department of Molecular Biology at the Max Planck Institute for Infection Biology.
This mechanism is vital after severe damage to the intestinal mucosa. Genetically modified mice that do not produce R-spondin did not show signs of ill health or changes to the epithelial structure under normal conditions. “This means that the stem cells can cope without R-spondin – other stem cell signals in their niche are able to compensate,” adds Thomas Meyer, Director at the Max Planck Institute for Infection Biology. However, colitis was fatal for these mice. Their remaining differentiated cells could no longer be reprogrammed to stem cells, leading to irreversible epithelial damage. These results now demonstrate what processes take place during colitis – and what mechanisms contribute to the recovery of the mucosa. They provide new directions for the development of therapies to treat patients with severe intestinal damage.
