Innate Immune Regulation
+++ The Majer Lab will move to the Cincinnati Children's Hospital Medical Center, Ohio, United States, in July 2025 +++
Immune responses require tight regulation to provide effective protection against infectious threats, while at the same time avoiding overwhelming inflammatory reactions. How immune cells achieve this balance at the molecular level is the overall focus of our research. We are taking a cell biological approach to study how the spatial subcellular organization and trafficking of innate immune receptors contributes to immune homeostasis. We aim to understand how perturbations of these processes promote inflammation and autoimmunity. To address these questions, we are using a combination of advanced imaging techniques, genome-editing and state-of-the-art protein perturbations, biochemical assays, and in vivo models.
Nucleic acid-sensing Toll-like receptors
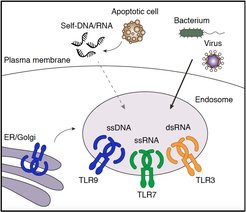
Receptors of the innate immune system recognize conserved microbial features and provide key signals to initiate an immune response. Multiple transmembrane and cytosolic immune receptors have evolved to recognize RNA and DNA, including members of the Toll-like receptor (TLR) family. Nucleic acid sensing enables the recognition of a broad range of pathogens, however, it also exposes the host to potential self-recognition and autoimmunity. A key mechanism to limit self-recognition by TLRs is their intracellular localization within endolysosomes. While endocytic and phagocytic uptake very efficiently delivers pathogens into endolysosomes for degradation, self-derived nucleic acids have only limited access to these compartments. Although endosomal localization is essential for proper self/non-self discrimination, very little is known about the actual spatial organization of TLRs within the endomembrane network, and which transport mechanisms and membrane interactions regulate receptor trafficking to their signaling compartments.
Mapping the Toll-Roads
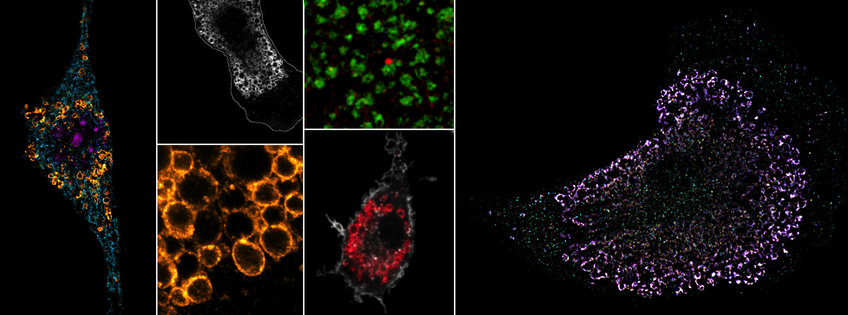
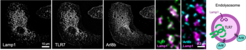
We want to map the precise locations of nucleic acid-sensing TLRs in high-resolution and identify the specific endosomes, in which ligand sensing and signaling occurs. We have previously shown that TLR7 (RNA sensor) and TLR9 (DNA sensor) are quite differently regulated within endosomes, opening up the possibility that these two receptors could signal from functionally distinct subsets of endosomes; an idea we are actively exploring. We also manipulate various aspects of endosome biology to probe how altered TLR trafficking and receptor turnover influences immune responses and self/non-self discrimination.
Organelle physiology

Endosomes are not only home to nucleic acid-sensing TLRs, but also serve as central metabolic coordinators and signaling hubs of the cell. Multiple environmental cues, such as starvation or sensing of pathogen-associated molecular patterns, induce remarkable architectural changes to the endomembrane system with corresponding functional adaptations. One example would be the transition from vesicular to tubulated endosomes in LPS-treated macrophages. We want to understand the relationship between organelle physiology/architecture and its impact on nucleic acid immunity. Through a variety of conditions, we induce endosome remodeling events in myeloid cells to interrogate the TLR signaling behavior within these different microenvironments. Our goal is to understand how the physiology of the endomembrane network shapes immune responses from within these organelles and how dynamic changes of the endosome architecture can contribute to inflammatory and autoimmune diseases.





