Neutrophil Extracellular Traps activate the immune system
Max Planck researchers show that Neutrophil Extracellular Traps (NETs) activate the immune response through the receptor cGAS
When neutrophils come in contact with pathogens, they eject NETs consisting of DNA and proteins that trap pathogens and activate the immune system. A team of researchers led by Arturo Zychlinsky now demonstrates that when the DNA in NETs are engulfed by phagocytes they activate the DNA receptor cGAS, which turns on the production of interferon. The results are now published in the journal Science Signaling.

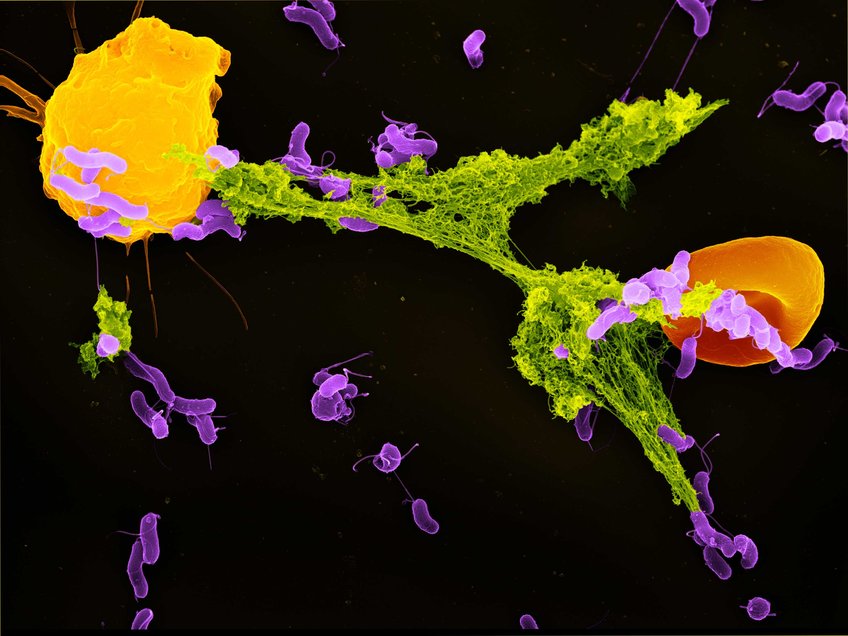
A neutrophil granulocyte (yellow) has ejected a NET (green) to capture bacteria (purple). A red blood cell (orange) is also trapped in the NET. The pathogens stick to the NET’s DNA threads and are killed by antimicrobial substances. A team of researchers led by Falko Apel now showed how NETs can activate the immune system in addition to this direct defense mechanism. Stained scanning electron microscope image by Volker Brinkmann.
Neutrophils are cells of the innate immune system. When pathogens invade our body, neutrophils identify and attack them. To do this, neutrophils have various defence mechanisms: they can ingest pathogens or secrete antimicrobials. Alternatively, when neutrophils come into contact with pathogens, they can disintegrate their cell and nuclear membrane and release network-like chromatin structures that are called neutrophil extracellular traps or NETs. NETs can trap and kill microbes and their remains are removed by phagocytes.
NETs activate cGAS – cGAS turns on interferon production
Previous studies showed that the remnants of NETs activate the immune system. Falko Apel, in the team of Arturo Zychlinsky at the Max Planck Institute for Infection Biology, got to the bottom of this signaling mechanism: they showed that NETs bind to the DNA receptor cGAS after uptake by phagocytes—thus stimulating the phagocytes to release pro-inflammatory signals, so called interferons, that trigger the immune response.
To understand the mechanism behind how NETs activate cGAS, the researchers first isolated neutrophils and phagocytes from healthy donors. In this controlled environment, they observed that the phagocytes respond to the production of NETs and release interferon. Following this result, the team wanted to find out which receptors in or on the phagocytes recognize the NETs. To do this, they genetically modified phagocytes, using the CRISPR/Cas9 system, and switched off receptors that recognize DNA, the main component of NETs. The DNA receptor cGAS was a hit: when the researchers inactivated this receptor, the phagocytes no longer reacted to NETs.
How do NETs and cGAS come together?
The subsequent experiments searched for clues. "NETs form extracellularly―but cGAS is inside the phagocytes," said Falko Apel, first author of the study. "That's why we wanted to find out how cGAS and NETs come together." In their experiments, the scientists traced the path of NETs: phagocytes take up DNA remnants in their phagosome, a cell compartment that digests cell remains. But instead of being digested, the NETs escape into the cell interior, where cGAS detects them. The receptor then triggers the production of type I interferons—pro-inflammatory signaling substances that activate the immune system. Since neutrophils are often the first immune cells to respond to pathogens, activation of the immune system recruits more cells to the defense.
The dark side of immune activation
However, the pro-inflammatory effect of NETs also has downsides—the activation of the immune system by NETs is linked to autoimmune diseases. These are diseases where the immune system turns against its own body and triggers chronic inflammation, such as systemic lupus erythematosus or rheumatoid arthritis. Indeed, patients with these diseases have increased levels of NETs and type I interferons.
This is particularly significant in the findings published by Apel and his coworkers; they have uncovered a fundamental link between NETs and the release of type I interferons. Their results help explain the negative side of NETs in chronic inflammation and might create a new approach to therapies. Until now, therapies rely on broad suppression of the immune system which have many side effects. New therapies targeting NETs or cGAS might specifically prevent the unwanted activation of the immune system.